How to characterize protein quality?
25 June 2019
Proteins are essential for our body. While being mainly known for their role as fundamental building blocks for muscles, these proteins compose only half of those present in the body. Skins, hair, sinews, some hormones, etc. all of these are composed of proteins. And proteins have many other applications in the body.
Are some protein sources better than others?
Yes there are. All the proteins in our diet are not equivalent. Each and every one is composed of a different amino acid chain and has its own structure. Therefore the quality of certain proteins will be a better match to our bodily needs thanks to their high digestibility and amino acids composition1.
How do we know which proteins are better?
The first thing to look at is the amino acids composition of the protein: the bigger the ratio of essential amino acids A is, the better the nutritional interest is.
The Chemical Score (CS) is used to evaluate this parameter. It is defined by comparing the essential amino acids ratio of the studied protein with the reference protein of the FAO1, which is considered as “ideal”: it contains all essential amino acids in the minimum necessary proportion for the body. Consequently its chemical score has been fixed at 1002.
To calculate the chemical score, we have to consider the essential amino acid with the lowest concentration and compare it with the reference protein of the FAO. So only the amino acid called the limiting amino acid is concerned.
A chemical score below 100 means that the studied protein has at least one essential amino acid present in insufficient number. Meanwhile if all essential amino acids are presents in higher quantities than the minimal bodily needs, then the chemical score will be higher than 100. It demonstrates a high quality protein1 2.
We can be more accurate in the essential amino acids composition study by looking at the BCAAB concentration. They are approximately 35% of muscles essential amino acids 3. An therefore they are necessary to protein muscle synthesis. Moreover, BCAA can be used as an energy source by muscles, especially during an intense effort4. As a result, a person’s diet has to provide enough BCAA, even more so for athletes 5.
So, does only essential amino acids composition define protein quality?
No it does not. Another parameter is important to consider: the protein digestibility. It corresponds to the amount of ingested protein which is effectively available for the body after digestion and absorption.
Digestibility can be measured in the ileum (small intestine last section) or in the feces (cf. figure 1). The first method is more accurate if we want to know the real amount of amino acids absorbed, but it is harder to use in Humans. Therefore the second method is more in use2.
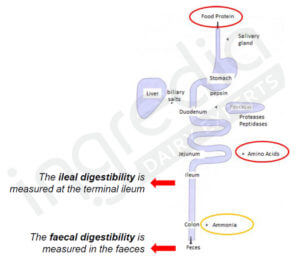
Figure 1: Ileal and faecal digestibility2
So we have two different data which give different information… Is their any indicator that would combine both information?
Yes there is, and it is called the PDCAAS (Protein Digestibility-Corrected Amino Acid Score). This indicator has been published by the FAO in 1991 and it considers essential amino acids composition and faecal digestibility 1 2:
The PDCAAS is scaled from 0 (bad protein quality and/or indigestible) and 1 (high quality and digestible protein). If a result is higher than 1, then it is set to 1.
All amino acids do not have the same digestibility. Is there a more accurate indicator?
This is true; each amino acid is assimilated differently by the body. That’s why the FOA has published a new indicator in 2013, the DIAAS (Digestible Indispensable Amino Acid Score). It considers the ileal digestibility of each amino acid, which is more accurate than the global faecal digestibility used for the PDCAAS 1 2.
What are the best proteins?
We need to distinguish proteins by their origin: from plants and from animals.
Animal based proteins usually have a well balanced essential amino acids profile and consequently a high chemical score. On the opposite, plant based proteins have often one or several limiting amino acids, reducing their chemical score. Figure 2 give some examples of chemical scores2 6 7 8 9.


Figure 2: Example of chemical scores of proteins 2 6 7 8 9
As far as the digestibility is concerned, plant based proteins (70 to 90%) usually have a lower score than animal based proteins (91 to 99%)2. As a consequence, if we consider the PDCASS or the DIAAS which include essential amino acids composition of the protein and its digestibility, it is not a surprise if the protein quality indicator is higher for animal based proteins than for plant based proteins (table 1).
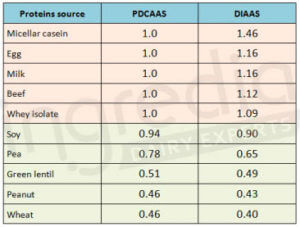
Table 1: Ranking of different protein sources based on PDCAAS and DIAAS 1 10 11 12 13 20 21
All of this is very technical… What are the pros and cons of each protein.
First, let’s have a look at animal based proteins. They have a great amino acids composition. So they are interesting for every category of people: the average adults, the growing children, the athletes who would like to improve their performances, and also the elderlies who want to maintain their muscle mass. These proteins are particularly interesting for these last two categories, since certain studies show that they are more efficient to stimulate muscle protein synthesis and to reduce protein breakdown 6 14 15. The drawback of animal based proteins can be found in meat, fishes and eggs, is that these products, with the exception of milk, are often sources of fat, more particularly saturated fats and cholesterol which excessive consumption can lead to adverse long-term health effect 6.

Plant based proteins often have one limiting amino acid. This is the reason why it is recommended for vegetarians and vegans to diversify their protein sources for their diet to provide all essential amino acids. Globally, leguminous plants (lentils, peas, …) have a sulfur amino acids deficit (Methionin and Cystein) and cereals do not have enough Lysin. Combining these two protein sources can be a good strategy. The main advantage of plant based proteins is that vegetables are a good sources of dietary fibers, vitamins and minerals, which have beneficial health effects. Another interesting plant protein source is soy because it presents a good amino acids profile and a good digestibility. It could be a protein able to compete with animal based proteins, however soy contains isoflavones, a molecule which mimic the actions of estrogens, a gender related hormone mainly present in female anatomy. Therefore in high amount, isoflavones can potentially lead to hormonal disorders. This risk caused certain studies to recommend avoiding the use of soy products for child nutrition 2 6 11 16

The best compromise would seem to be milk proteins: whey and micellar caseins. Like the other animal proteins, they present a well balanced amino acids composition and are a good source of BCAA. Moreover they contain a lot of bioactive peptides – i.e. short amino acids chains which have biological effects such as stress reduction 6 17 18. Digestibility-wise, our body reacts differently to whey and micellar caseins. As a result they are complementary 19.
Today, the easy access to large food diversity allows us to select product with the best quality. So for proteins it is important to understand which parameters can define their quality. This allows us to know what the best proteins are for our needs and our lifestyle. Regarding milk proteins, Ingredia is committed to the use of the best raw material and the least denaturing processes. In order to better preserve the native structure of the proteins.
For more information, please contact us
Authors: Rémi Maleterre & Audrey Boulier.
*A [Essential Amino Acids]: Amino acid that the body cannot synthesize. Therefore they must be supplied through the diet.
*B [BCAA, Branched-Chain Amino Acids]: Essential amino acids which correspond to Leucine, Isoleucine and Valine.
_________________________________________________________________________________________
[1] Report of an FAO Experte consultation. Dietary protein quality evaluation in human nutrition. ISSN 0254-4725 FAO and food nutrition paper 92. 31 March – 2 April, 2011.
[2] D. Tomé (2018 – Lille, France) – Protein Digestibility Scores & Nutrition, AgroParisTech – Departement of Life Sciences and Health; INRA, UMR Nutrition physiology and ingestive behavior.
[3] A.E. Harper, R. H. Miller, K. P. Block (1984). Branched-Chain Amino Acid Metabolism. Annual review of Nutrition. Vol. 4:409-454. Epub July, 1984. https://doi.org/10.1146/annurev.nu.04.070184.002205
[4] M. J. Rennie (1996). Influence of Exercise on Protein and Amino Acid Metabolism. Handbook of Physiology, exercise: regulation and integration of multiple systems. Epub January, 2011. https://doi.org/10.1002/cphy.cp120122
[5] Y. Shimomura, T. Murakami, N. Nakai, M. Nagasaki, R. A. Harris (2004). Exercise Promotes BCAA Catabolism: Effects of BCAA Supplementation on Skeletal Muscle during Exercise, The Journal of Nutrition, Volume 134, Issue 6, Pages 1583S–1587S. Epub Octobor 01, 2004. https://doi.org/10.1093/jn/134.6.1583S
[6] J.R. Hoffman, and M.J. Falvo. (2004). Protein – Which is Best?. Journal of sports science & medicine vol. 3,3 118-30. Epub September 01, 2004.
[7] Protein Quality Evaluation, Report of the Joint FAO/WHO Consultation. Expert Consultation Bethesda, Md., USA. 4-8 December 1989.
[8] Ingredia. Prodiet 87 B Fluid – Nutrition facts. December 11, 2015.
[9] Ingredia. Prodiet 90 S – Nutrition facts. March 18, 2016.
[10] J.K. Mathai, Y. Liu, H.H. Stein (2017). Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). Br J Nutr. 117(4):490-499. Epub February, 2017. https://doi.org/10.1017/S00D7114517000125
[11] M. Jarpa-Parra (2017). Lentil protein: a review of functional properties and food application. An overview of lentil protein functionality. International Journal of Food Science & Technology. Vol 53, issue 4. Epub November 21, 2017. https://doi.org/10.1111/ijfs.13685
[12] S.M. Rutherfurd, A.C. Fanning, B.J. Miller, P.J. Moughan (2015). Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J Nutr. 145(2):372-9. Epub February, 2015. https://doi.org/10.3945/jn.114.195438
[13] M.G. Nosworthy, J. Neufeld, P. Frohlich, G. Young, L. Malcolm, J.D. House (2017). Determination of the protein quality of cooked Canadian Pulses. Food Science & Nutrition. Epub June 20, 2017. https://doi.org/10.1002/fsn3.473
[14] W.W. Campbell, M.L. Barton Jr., D. Cyr-Campbell, S.L. Davey, J.L. Beard, G. Parise, W.J. Evans (1999). Effects of an omnivorous diet compared with a lactoovovegetarian diet on resistance-training-induced changes in body composition and skeletal muscle in older men. American Journal of Clinical Nutrition 70, 1032-1039. Pub December 01, 1999. https://doi.org/10.1093/ajcn/70.6.1032
[15] D.L.E. Pannemans, A.J.M. Wagenmakers, K.R. Westerterp, G. Schaafsma, D. Halliday (1998). Effect of protein source and quantity on protein metabolism in elderly women. American Journal of Clinical Nutrition 68, 1228-1235. Pub December 01, 1999. https://doi.org/10.1093/ajcn/68.6.1228
[16] C.R. Cederroth, J. Auger, C. Zimmermann, F. Eustache, S. Nef (2010). Soy, phyto-oestrogens and male reproductive function: a review. International Journal of Andrology, Vol 3, Issue 2. Epub March 14, 2010. https://doi.org/10.1111/j.1365-2605.2009.01011.x
[17] C.M. Kerksick, C.D. Wilborn, M.D. Roberts, A. Smith-Ryan, S.M. Kleiner, R. Jäger, R.Collins R., Cooke M., Davis J.N., Galvan E., Greenwood M., Lowery L.M., Wildman R., Antonio J. and Kreider R.B. (2018). ISSN exercise & sports nutrition review update: research & recommandations. Journal of the International Society of Sports Nutrition, 15:38. Epub August 01, 2018. https://doi.org/10.1186/s12970-018-0242-y
[18] Y.W. Park, M.S. Nam (2015). Bioactive peptides in milk and dairy products: a review. Korean Journal for Food Science of Animal Resources, 35: 831–840. Epub December 31, 2015. https://doi.org/10.5851/kosfa.2015.35.6.831
[19] M. Lacroix, C. Bos, J. Léonil, G. Airinei, C. Luengo, S. Daré, R. Benamouzig, H. Fouillet, J. Fauquant, D. Tomé, C. Gaudichon (2018). Compared with casein or total milk protein, digestion of milk soluble proteins is too rapid to sustain the anabolic postprandial amino acid requirement. Am J Clin Nutr 2006; 84:1070–9/ Epub January 23, 2018. https://doi.org/10.1556/1646.10.2018.10.
[20] A. Ogawa, H. Murayama, K. Hayamizu, Y. Kobayashi, M. Kuwahata, Y. Kido (2015). A simple evaluation method for the quality of dietary protein in rats using an Indicator Amino Acid Oxidation technique. J Nutr Sci Vitaminol, 61(2):123-30. Pub 2015. https://doi.org/10.3177/jnsv.31.123
[21] R.R. Wolfe (2015). Update on protein intake: importance of milk proteins for health status of the elderly. Nutrition Reviews, 73(Suppl 1):41-47. Pub August 2015. https://doi.org/10.1093/nutrit/nuv021